The Exclusion Principle –
IYQ25
Pioneers of Quantum Theoretical Physics
Part 6
Wolfgang Pauli
UNESCO has proclaimed 2025 as the International
Year of Quantum Science and Technology (IYQ). This year-long,
worldwide initiative will celebrate the contributions of quantum science to
technological progress over the past century, raise global awareness of its
importance to sustainable development in the 21st century, and ensure that all
nations have access to quantum education and opportunities.
In celebration of IYQ25, this series of articles focuses on the key personalities of quantum theoretical physics and their work – ten of the greatest, from Planck to Feynman. This is the sixth article in the series and focuses on Pauli and his Exclusion Principle which is foundational to the structure of all matter. For the earlier articles in this series, see 1,2,3,4,5.
Introduction
In order to understand the Pauli Exclusion Principle (PEP), we first need to review some basic quantum physical concepts:
Atomic Orbital: This is a region in an atom where an electron is most likely to be found. These orbitals are described by mathematical functions and have specific shapes and energy levels. An orbital can hold a maximum of two electrons.
The
shapes of the first five atomic orbitals are 1s, 2s, 2px, 2py,
and 2pz. The two colours show the phase or sign of the wave function
in each region.
Quantization: Quantum systems exhibit quantized properties, meaning certain physical quantities like energy, momentum, and angular momentum can only take discrete values, unlike classical systems where they can vary continuously. A quantum system can exist in a combination of multiple states simultaneously. This is the essence of the superposition principle. Measurements of quantum systems yield probabilistic outcomes, meaning that the result of a measurement is not predetermined but a probability distribution.
Quantum Numbers: Quantum numbers are quantities that characterize the possible states of a system. To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed as illustrated in the diagram below:
Fermions and bosons: Fermions (named after the Italian physicist Enrico Fermi) and bosons (named after India’s Satyendra Nath Bose) are two fundamental classes of particles, distinguished by their intrinsic angular momentum, also called spin. Fermions have half-integer spins (1/2, 3/2, etc.) while bosons have integer spins (0,1,2, etc.). These differences in spin lead to vastly different behaviors and roles in the universe).
Electrons, protons and neutrons are common examples of fermions. The neutrino, first predicted by Pauli, is also a fermion. As we shall see later, these particles obey the Pauli Exclusion principle, meaning no two identical fermions can occupy the same quantum state. Combinations of an odd number of fermions are also fermions. Fermions make up the matter in the universe.
Photons, gluons, Higgs bosons, W and Z bosons are some examples of bosons, which have integer spin (0,1,2, etc.). They do no obey the Pauli principle, implying that multiple bosons can occupy the same quantum state. Combinations of an even number of fermions, like some atoms and molecules, can be bosons.
Wave Function & Probability: Quantum particles are described by a wave function (Ψ). The square of its magnitude (|Ψ|²) gives the probability density of finding the particle at a specific location and time.
Cross-sections of
atomic orbitals of the electron in a hydrogen atom at different energy levels. The
probability of finding the electron is given by the colour, as shown in the key
at upper right.
Indistinguishability: For identical particles (like all electrons), it is fundamentally impossible to label or track which particle is which. Any measurement can only reveal "an electron" at a point, not "electron A" or "electron B".
Wave Function, Symmetry: Due to indistinguishability, the multi-particle wave function must behave in a specific way when the coordinates (position and spin) of any two identical particles are swapped. For bosons the wave function remains unchanged (symmetric): Ψ(1,2) = +Ψ(2,1). For fermions (half-Integer spin) the wave function changes sign (antisymmetric): Ψ(1,2) = -Ψ(2,1)
Antisymmetry and Exclusion: The antisymmetry requirement for fermions is the mathematical root of the Pauli Exclusion Principle. Imagine two fermions trying to occupy the exact same quantum state (same set of quantum numbers). Their wave function would be Ψ(1,2) = φₐ(1)φₐ(2), where φₐ is the single-particle state. Swap the particles: Ψ(2,1) = φₐ(2)φₐ(1) = φₐ(1)φₐ(2) = Ψ(1,2). But antisymmetry requires Ψ(2,1) = -Ψ(1,2). The only way Ψ(1,2) = -Ψ(1,2) can be true is if Ψ(1,2) = 0! Ψ = 0 means the probability of finding the two particles in that identical state is exactly zero. They are mutually excluded. This forces fermions into different states.
The historical context
In the early 1920s, atomic spectroscopy presented puzzles that existing quantum theory (the Bohr-Sommerfeld model) couldn't resolve:
· Anomalous Zeeman Effect: The splitting of spectral lines in magnetic fields
was more complex than predicted.
· Alkali Metal Spectra: Why did these elements (Li, Na, K...) have spectra resembling hydrogen
but with more complex doublet structures?
· Shell Structure & Periodic Table: How could electrons be arranged to explain the
periodic table? Why didn't all electrons collapse into the lowest energy state?
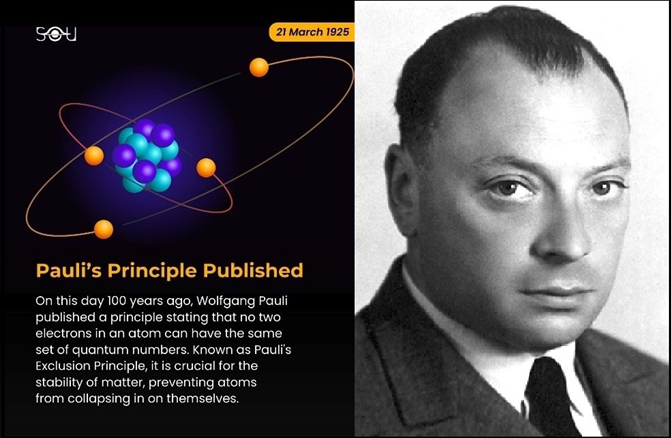
Pauli tackled these problems head-on. He analyzed data from atomic spectra, particularly focusing on the Zeeman effect and alkali metals. In 1924, he proposed the idea of a "classically non-describable duplexity" – a mysterious two-valuedness in the electron's quantum properties that wasn't related to spatial motion. This was his key insight before the concept of electron spin was formally introduced. In 1925, Pauli published his famous paper "Über den Zusammenhang des Abschlusses der Elektronengruppen im Atom mit der Komplexstruktur der Spektren" (On the Connection between the Completion of Electron Groups in an Atom and the Complex Structure of Spectra). Here he stated his principle: "There can never be two or more equivalent electrons in an atom for which... the values of all quantum numbers... are the same. If an electron exists in an atom for which these quantum numbers... have definite values, then this state is 'occupied'.
Motivation & Insight: Pauli realized that to explain why atomic electrons didn't all crowd into the lowest orbit (n=1), and to account for the observed spectral lines and chemical periodicity (as evident in the periodic table of elements), an additional rule was needed beyond the quantum numbers n, l, mₗ. He postulated a fourth quantum number (later identified as electron spin, projection mₛ = ±1/2) and the rule that no two electrons in an atom can share the same full set of four quantum numbers (n, l, mₗ, mₛ). This explained the shell structure (2 electrons per orbital) and the periodic table's periods.
While Pauli initially framed it with his "duplexity," he was famously reluctant about Uhlenbeck and Goudsmit's proposal of electron spin (also in 1925/26) as a physical rotation. However, the fourth quantum number was soon directly linked to the intrinsic angular momentum (spin) and its projection on the spin axis (mₛ). Dirac's relativistic quantum theory (1928) later fundamentally derived the connection between spin and particle statistics.
PEP and the Periodic Table of Elements
The Pauli Exclusion Principle forces electrons to occupy progressively higher energy levels and orbitals as atomic number increases. This systematic, step-wise filling of orbitals with strictly defined capacities is what creates the repeating patterns of electron configurations in the outermost shells, leading to the periodic repetition of chemical properties that forms the very foundation of the periodic table. It dictates the periods, groups, blocks, and ultimately the chemical behavior of the elements.
Wolfgang Pauli (1900 - 1958) – A biographical sketch
Wolfgang Pauli (1900-1958) was one of the most brilliant and influential theoretical physicists of the 20th century, whose foundational contributions fundamentally shaped our understanding of quantum mechanics and atomic structure. His work provided the scaffolding upon which modern particle physics and quantum chemistry were built, earning him the 1945 Nobel Prize in Physics and the enduring title "conscience of physics" for his penetrating critical insights.
Born in Vienna on 25 April 1900, Wolfgang Ernst Pauli was immersed in intellectual ferment from infancy. His father, Wolfgang Joseph Pauli, was a distinguished professor of colloid physics at the University of Vienna, while his mother, Berta Schütz, was a writer connected to Vienna's cultural elite. Significantly, the physicist-philosopher Ernst Mach served as Pauli's godfather and namesake (Pauli's middle name "Ernst" honored Mach), profoundly influencing his epistemological outlook with what Pauli later described as an "anti-metaphysical baptism".
Pauli
as a school boy
Pauli's exceptional mathematical abilities manifested early. While attending the Döblinger-Gymnasium, he taught himself Einstein's general relativity—a revolutionary theory at the time—and published his first scientific paper on the subject at age 18, shortly after graduating in 1918. This early mastery foreshadowed his future impact. At the University of Munich, he studied under the legendary Arnold Sommerfeld, who recognized Pauli's genius and tasked the 20-year-old with writing the encyclopedia entry on Relativity for the Encyklopädie der mathematischen Wissenschaften. The resulting 237-page monograph, completed just months after Pauli earned his doctorate in 1921, became a classic on the subject. Albert Einstein praised it effusively ("No one studying this mature, grandly conceived work would believe that the author is a man of twenty-one...").
In 1921, Pauli got his doctoral degree, with a thesis on the quantum theory of H₂⁺. During 1921-23, he had assistantships to Max Born and Niels Bohr, two of the pillars of quantum physics. He worked mostly on atomic spectra; this soon led to his Exclusion Principle.
Pauli's return to Hamburg as a lecturer in 1923 coincided with the quantum theory's profound crisis. Physicists struggled to explain "anomalous Zeeman effect"— the puzzling splitting of spectral lines in magnetic fields that defied existing quantum models. Pauli spent two intense years wrestling with this problem. His breakthrough emerged from combining meticulous spectral analysis with a radical conceptual leap. Building on British physicist Edmund Stoner's work on electron distribution, Pauli realized atomic electrons required a fourth quantum number possessing "two-valuedness not describable classically”.
Pauli with Paul Ehrenfest
In 1925, he formulated the Exclusion Principle: No two electrons in an atom can occupy the same quantum state simultaneously - meaning they cannot share identical values for all four quantum numbers. This principle elegantly explained
electron shell structure (why electrons occupy
distinct energy levels rather than collapsing into the lowest state) and the periodic
table organization, the underlying reason for chemical periodicity and valence,
as also matter's stability (the fundamental reason why solid matter doesn't
implode).
Initially resistant to interpreting his fourth quantum number as electron spin (proposed by Uhlenbeck and Goudsmit in 1925), Pauli later incorporated spin into his formalism, developing the Pauli matrices (1927) - a 2x2 matrix representation essential for describing spin -½ particles quantum mechanically. The exclusion principle became the cornerstone of Fermi-Dirac statistics, governing all fermions (as did Bose-Einstein statistics for all bosons).
The Neutrino Hypothesis and Quantum Field Theory
While at the Swiss Federal Institute of Technology (ETH) in Zurich (1928 onwards), Pauli tackled another crisis: beta decay energy conservation. Observations showed energy appeared lost when atomic nuclei emitted electrons (beta particles), seemingly violating sacred conservation laws. In a bold 1930 letter addressed playfully to "Dear Radioactive Ladies and Gentlemen" at a Tübingen conference, Pauli proposed a radical solution: an uncharged, massless (or nearly massless) particle emitted alongside the electron, carrying off the "missing" energy and momentum.
He initially called this hypothetical particle a "neutron," but after Chadwick's discovery of the nuclear neutron (1932), Enrico Fermi renamed it the neutrino ("little neutral one"). Pauli worried his idea was "something no theorist should ever do"—postulating an undetectable particle—yet it preserved fundamental physics principles. Experimental confirmation came only in 1956 (by Reines and Cowan). Pauli famously telegraphed: "Thanks for message. Everything comes to him who knows how to wait”.
In 1940, Pauli derived the profound spin-statistics theorem, proving a fundamental connection between a particle's intrinsic spin and its quantum behavior: fermions (half-integer spin) obey the exclusion principle, while bosons (integer spin) do not. This theorem underpins all quantum field theory. Post-WWII, his work on renormalization in quantum electrodynamics and proving the CPT theorem (charge-parity-time symmetry) further solidified quantum field theory's foundations.
Leadership, Exile, and Philosophical Quest
Pauli transformed ETH Zurich into a global hub for theoretical physics. His seminars with Gregor Wentzel and mentorship of future luminaries like Rudolf Peierls, Felix Bloch, and Markus Fierz fostered exceptional collaborations. However, the Nazi annexation of Austria (1938) made Pauli, despite his Catholic upbringing, a German citizen with Jewish ancestry, forcing his emigration to Princeton's Institute for Advanced Study in 1940, which had been haven to Einstein.
Personal crises—his mother's suicide (1927), a brief failed marriage (1929-1930)—led him to psychoanalysis with Carl Jung. Their collaboration evolved into a deep, decades-long dialogue exploring synchronicity and the psychic-physical unity of reality. Pauli wrote Jung: "It is my personal opinion that in the science of the future reality will neither be 'psychic' nor 'physical' but somehow both and somehow neither”. This philosophical shift distanced him from his early Machian positivism.
Nobel Recognition and Enduring Legacy
Awarded the 1945 Nobel Prize for the exclusion principle, Pauli received U.S. citizenship in 1946 but chose to return to Zurich, later becoming a Swiss citizen (1949). He continued groundbreaking work until his death from pancreatic cancer on December 15, 1958. Poignantly, he noted his hospital room number was 137—approximating the inverse fine structure constant, a dimensionless fundamental constant that fascinated him.
Pauli's dual legacy:
- Scientific: The exclusion principle governs
atomic structure, chemistry, and condensed matter physics. The neutrino is
central to the Standard Model and astrophysics. The spin-statistics theorem
underpins quantum field theory.
- Philosophical: His critique of scientific
epistemology and exploration of consciousness's role in physics resonate in
modern debates about quantum interpretation.
As Einstein declared, Pauli was his worthy scholarly successor—a thinker whose uncompromising search for quantum reality's architecture reshaped our universe's understanding at its most fundamental level. His work remains embedded in the bedrock of modern physics, a testament to the power of profound insight wedded to mathematical rigor.
Pauli with Einstein
Profound Implications of PEP for Quantum Science & Technology:
The Pauli Exclusion Principle is far more than just an atomic rule; it's a fundamental law governing the behavior of matter:
- Structure of Matter: Atomic Structure & Chemistry: PEP dictates electron shell filling (2n² electrons per shell), explaining the periodic table, chemical bonding, valence, and the very existence of diverse elements and molecules. Without PEP, all electrons would collapse to the 1s orbital, and chemistry as we know it wouldn't exist.
- Solid-State Physics: PEP governs electron behavior in solids, leading to Band Structure - formation of energy bands and band gaps (crucial for understanding conductors, semiconductors, and insulators).
- Fermi Energy/Gas: The concept of a Fermi surface and Fermi energy describing the highest occupied electron state at absolute zero.
- Semiconductor Technology: The basis for diodes, transistors, integrated circuits, LEDs, lasers, solar cells – the foundation of modern electronics and computing.
- Magnetism: Pauli paramagnetism and understanding ferromagnetism rely heavily on PEP and spin.
- Nuclear Structure: Governs the arrangement of protons and neutrons in atomic nuclei, explaining nuclear magic numbers and stability.
- Astrophysics:
- White Dwarf Stars: Electron degeneracy pressure, arising directly from PEP preventing electrons from occupying the same state, counteracts gravitational collapse.
- Neutron Stars: Neutron degeneracy pressure (also from PEP) is the primary force preventing these incredibly dense objects from collapsing into black holes.
- Quantum Statistics: PEP is the foundation of Fermi-Dirac statistics, which describes the behavior of systems of identical fermions (e.g., electrons in a metal, electrons in a white dwarf). This contrasts sharply with Bose-Einstein statistics for bosons.
- Advanced Quantum Phenomena: Quantum Hall Effects: PEP plays a crucial role in explaining the quantization of the Hall resistance.
- Lasers: While lasers rely on bosonic stimulation (photons are bosons), PEP is essential for understanding the population inversion process in the gain medium (electrons are fermions).
- MRI/NMR: Nuclear spins (fermions) obey PEP, influencing their energy levels in magnetic fields, which is fundamental to magnetic resonance imaging and spectroscopy.
- Quantum Information Science. PEP governs the state occupation of qubits based on fermions (e.g., electron spins or quantum dots).
- It underlies concepts like exchange interaction and entanglement in fermionic systems.
- Fundamental Physics: PEP, combined with the Spin-Statistics Theorem (which links particle spin to its quantum statistics), is a foundational pillar of relativistic quantum field theory (QFT). Pauli himself used the principle (and conservation of energy/momentum) to predict the existence of the neutrino in 1930 to explain beta decay spectra.
In Summary:
The Pauli Exclusion Principle arises from the fundamental requirement of antisymmetry in the wave function of identical fermions. Pauli deduced it from the need to explain atomic spectra and the periodic table, postulating a fourth quantum number (spin) and the exclusion rule. Its implications are vast and foundational: it explains the structure of atoms, nuclei, and all matter; dictates the behavior of electrons in solids (enabling modern electronics); governs the stability of stellar remnants; defines quantum statistics; and is deeply embedded in the theoretical framework of particle physics and quantum field theory. Without PEP, the universe as we know it – with complex atoms, diverse chemistry, solid materials, stars, and planets – simply could not exist.
1 comment:
I always learn something from these blogs even if I am familiar with the Physics. I did not know the story behind the neutrino till now.
You have showcased the magnificence of the PEP so clearly sir - a lot of modern Physics and Technology wouldn't exist without it.
Post a Comment